Pipeline
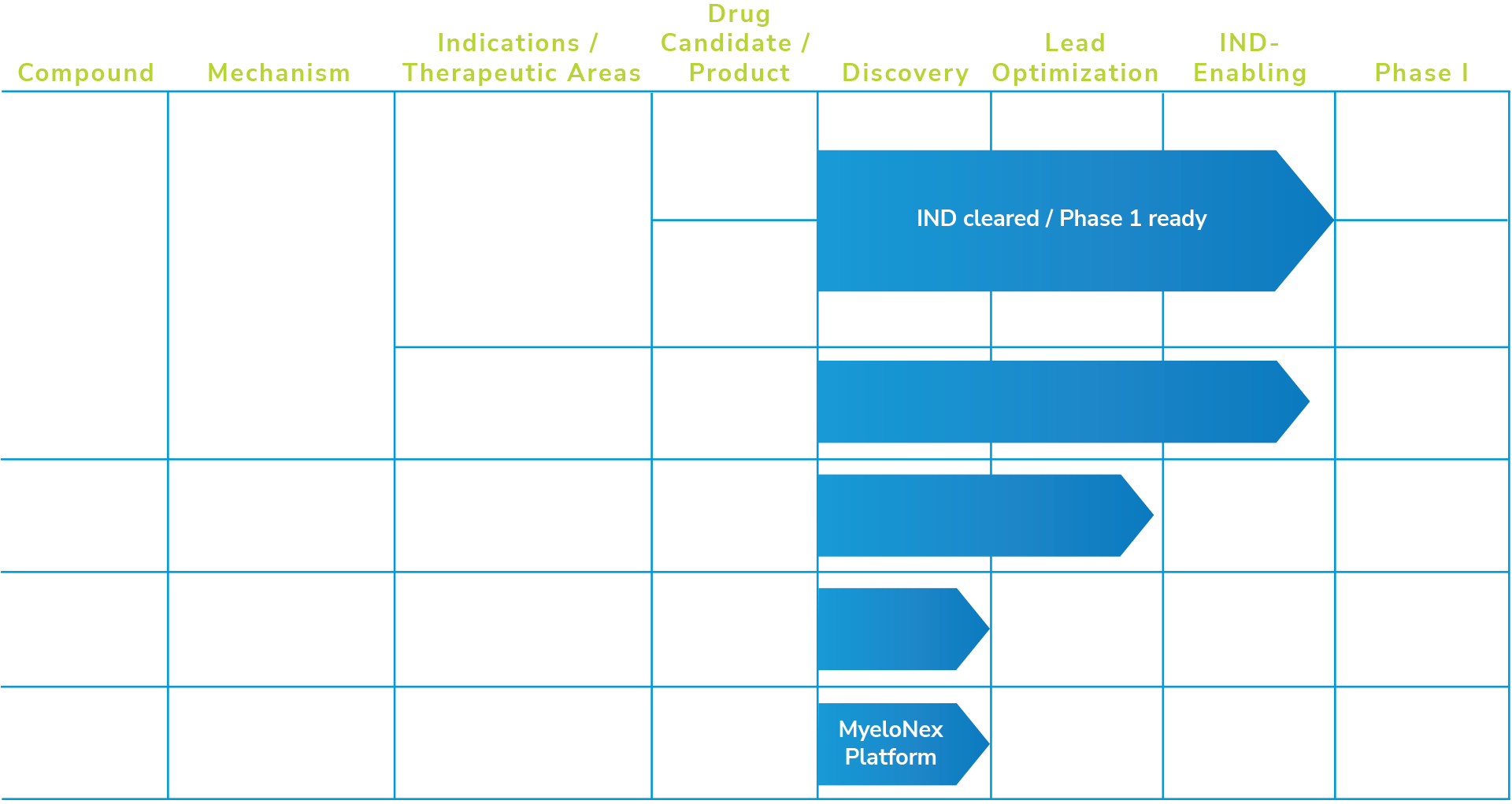
AVS100 – Highly Selective HDAC6i
(Small Molecule)
AVS100 is a highly selective, orally bioavailable HDAC6 inhibitor intended for the treatment of locally advanced or metastatic solid tumors, including in combination with existing PD-1 inhibitor pembrolizumab. In a majority of advanced solid tumors, response rates remain low such as in metastatic melanoma where only 20-30% of patients are responsive to existing therapies; highlighting substantial unmet need. One of the key correlates with patients who do not respond to existing therapy or progress regardless of standard of care options, are the presence of immunosuppressive cells in the tumor microenvironment (TME) such as tumor associated macrophages. Pro-tumoral macrophages and other myeloid suppressor cells can constitute up to 50% or more of solid tumor cell mass. AVS100 has been proven preclinically as published in Science Advancese to be able to suppress the polarization of tumoral macrophages towards pro-tumoral phenotypes, including in human cells. In preclinical in-vivo studies, AVS100 has substantial efficacy in reducing tumor growth as a monotherapy or in combination with anti-PD1 in multiple models, as well as increasing the overall M1/M2 macrophage ratio in the TME. AVS100 also has an exceptional preclinical safety profile, with no mutagenicity or cardiotoxicity and minimal GLP toxicology drug related findings. AVS100 has been IND cleared by U.S. FDA for conducting a Phase Ia/b trial targeting locally advanced or metastatic solid tumors in combination with pembrolizumab. Avstera intends to initiate this clinical study in the first half of 2025.
Next Generation HDAC6 Inhibitors
Avstera is exploring the potential of next generation, orally bioavailable and safe HDAC6 inhibitors to treat a variety of cardio-cardiometabolic indications such as obesity. Novel HDAC6i has the potential to act as suppressors of adipogenesis, total lipid reducers, and cardio protective agents with broad applicability. The company intends to advance lead candidates this year to IND-enabling studies.
MyeloNex™ Discovery Platform
This is a platform that will allow the discovery and validation of novel molecular targets in myeloid cells, including macrophages, dendritic cells, neutrophils, and others that regulate their function.
The technology corresponds to proprietary human myeloid hematopoietic progenitors that have been transitionally immortalized. These differentiated cells are identical to primary cells obtained from healthy donors and therefore a model system to study their function and for cell therapy applications.




